Our Expertise
SaMD Development, Regulatory-Compliant and x10 Cheaper
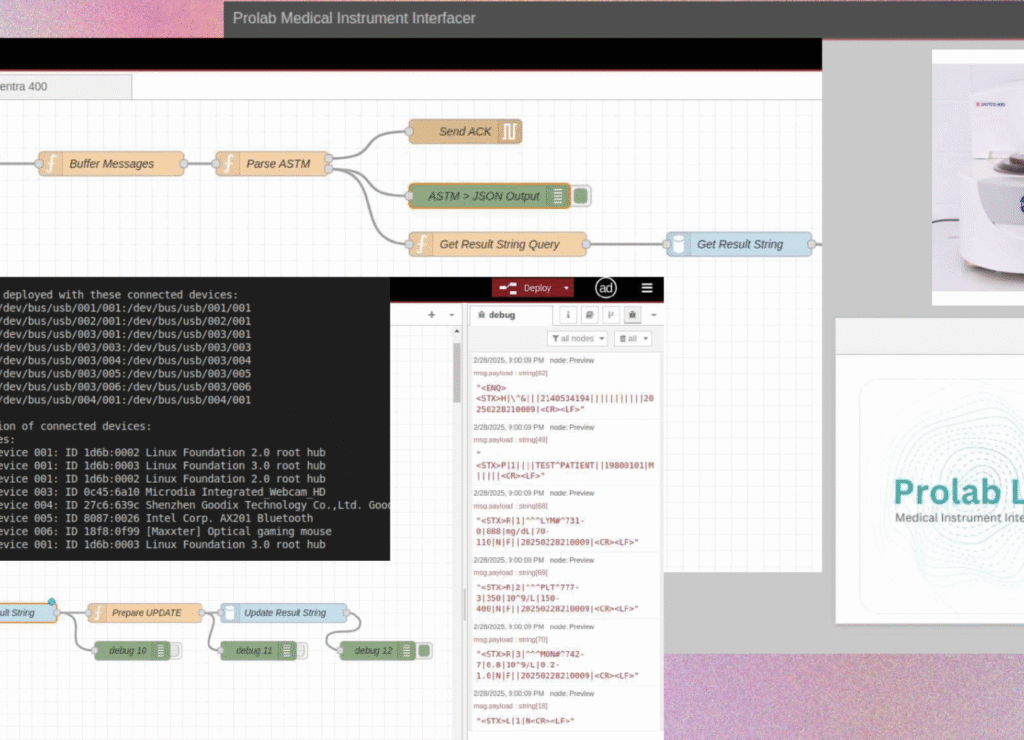
We specialise in regulatory-compliant SaMD (Software as a Medical Device) and innovative AIaMD (Artificial Intelligence as a Medical Device) solutions. We help healthcare providers and innovators accelerate their digital health journey by combining medical software design expertise, healthcare data integration and a deep understanding of international standards, including ISO 13485, IEC 62304, and the Medical Device Regulation (MDR). Our mission is to deliver secure, interoperable, and intelligent medical software that transforms patient care and enables organisations to bring cutting-edge technologies to the UK market.